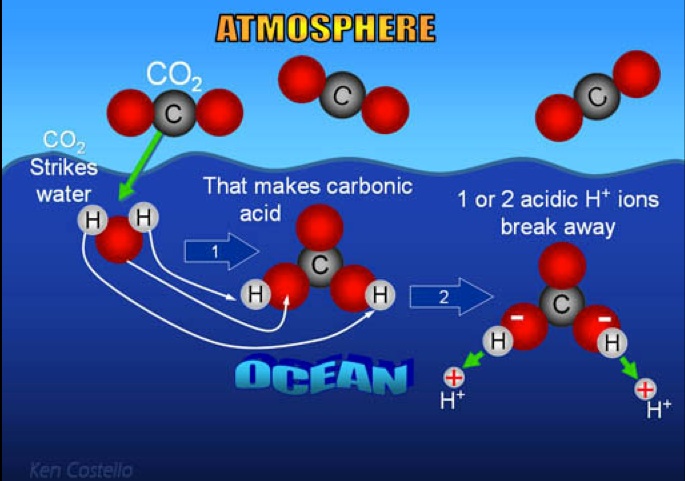
With all the attention given to global warming, a second potentially
devastating effect of the human addition of carbon dioxide to the air has
rather slipped under the radar – the increasing acidity of the oceans.
The oceans have probably saved us from the worst effects
of global warming already, by soaking up huge amounts
of the carbon dioxide pumped into the air by human activity.
Estimates vary, but anything between a third and a half of all
the carbon dioxide added has been absorbed by the oceans. This
ocean buffer comes at a cost. Carbon dioxide does not simply
disappear when it goes into the oceans; it alters the chemistry of
the oceans, turning them slightly acidic.
Acidity is measured on the pH scale that shows on a scale
of 0 to 14 how acid or alkaline a liquid is by the concentration
of hydrogen ions: 0 is the most extreme acid; 14 is the most
extreme alkali; 7 is neutral. Sea water is slightly alkaline, with a
pH ranging from 7.5 to 8.4. This alkalinity is important, because
numerous creatures from corals to pteropod shellfish rely on the
availability of carbonates in the water to build their shells and
skeletons, which are rich in calcium carbonates.
Dipped in acid
Acidity would have to rise dramatically before shells and
skeletons were actually dissolved in a nightmarish acid bath
scenario, but even a slight decrease in alkalinity could reduce
the availability of carbonates and seriously disrupt growth.
Experiments have shown that pH has to change by just 0.2 to
hamper the growth of sensitive creatures such as corals and
plankton. The ocean’s pH has already dropped by 0.1 since preindustrial
times. Scientists at the Carnegie Institution believe it
could fall by 0.35 over the next 50 years. The acidification could
be even more extreme in surface waters, where the vast majority
of marine life lives, because carbon dioxide is absorbed at the
surface. The focus so far has been on corals, which some marine
biologists suggest could be destroyed entirely around the world
by acidification in just a few decades – even if they survive other
environmental hazards. But the effects of acidification could
reverberate through the whole marine ecosystem. For a start, it
can affect calcification (the formation of bone and shell) for a
huge range of sea creatures. Young and developing organisms
are very vulnerable, since they need ample carbonates for
growth. If many species spend much longer in the larval stage,
they might become much more vulnerable to predation.
Acid trip
Acidification could also have a dramatic impact through
the damage it causes to key species, such as phytoplankton.
Phytoplankton build themselves calcium carbonate shells
to protect them from microscopic predators such as ciliate
protozoa. If robbed of their carbonate armour, plankton could
be gorged on by protozoa, with the effects of their decline
rippling right the way through the food chain – and having even
more significant consequences as their huge addition of oxygen
to the environment is curtailed. Similarly, if pteropods decline
because they are unable to make their shells, their natural
predators may be forced to look elsewhere for food, which could
cause major disruption to fish populations.
Some believe that acidification may also be playing a part in
the decline of fish stock in coastal waters, where there are other
environmental problems. But with all the focus on the terrestrial
effects of greenhouse gases and climate change, the research into
the effects of ocean acidification are only just getting under way.
Yet there is genuine cause for concern. The acidification in the
oceans is far harder to turn around than atmospheric greenhouse
gases. To prevent the pH dropping to 0.2, the level at which
corals and many other creatures would be seriously threatened,
carbon dioxide emissions would have to be cut right now.
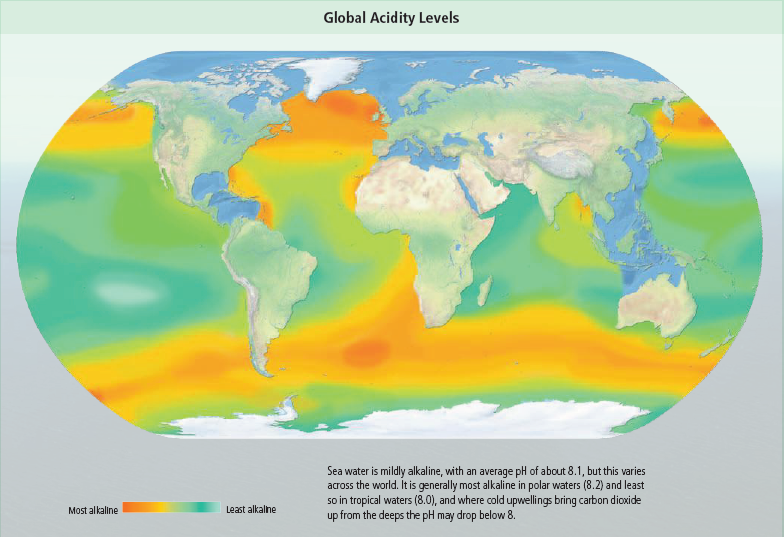
Prior to the Industrial Revolution, average ocean pH was about 8.2. Today, average ocean pH is about 8.1.Models consistently project further ocean acidification worldwide. Ocean surface pH is projected to decrease to values between 8.05 and 7.75 by the end of 21st century, depending on future CO2 emissions levels. The largest projected decline represents more than a doubling in acidity.
Normal rain has a pH of about 5.6; it is slightly acidic because carbon dioxide (CO2) dissolves into it forming weak carbonic acid. Acid rain usually has a pH between 4.2 and 4.4.